Boink Group – Cardiac rhythm regeneration
We are a translational research group integrated in the Departments of Medical Biology and Clinical Cardiology of the Academic Medical Center (AMC), University of Amsterdam. Our aim is to improve heart rhythm management by developing novel regenerative therapies to prevent or cure cardiac arrhythmias.
Contact: G. J. Boink (g.j.boink@amc.nl)
Cardiac arrhythmias are a major cause of morbidity and mortality. Current treatment options range from drugs and devices to more invasive therapies like ablation and surgery. Despite ongoing improvements in these treatments, important limitations remain, resulting in substantial lethality. To address these issues, we dedicate our research to the development of novel regenerative therapies to treat cardiac arrhythmias.
1. Biological Pacing
More than 700,000 pacemakers are implanted in the United States and Europe annually. These devices save many lives, but in about five percent of the implants serious complications arise that require surgery or other invasive procedures. Moreover, electronic pacemakers harbor important shortcomings. These electronic devices are not directly sensitive to autonomic modulation, resulting in a suboptimal heart rate response to exercise and emotion. In addition, limited battery life requires device replacement every five to ten years. Electronic pacemaker limitations are particularly problematic in pediatric patients, who face multiple (> 10) reimplantation procedures during their lives, with increasing risk for complications.
Our research focuses on developing alternatives that would overcome these disadvantages: that is, biological pacemakers constructed using gene and/or cell therapy. These pacemakers can be introduced following minimally-invasive procedures without requiring major surgery. Transplantation of differentiated stem cells can be used to regenerate the pacemaker and conduction system of the heart. Alternatively, viral vectors can be employed to modify and locally repair diseased cells. Figure 1 summarizes some of the most potent viral gene transfer strategies explored so far. We are currently developing these strategies towards clinical testing.
2. Repair of atrioventricular conduction
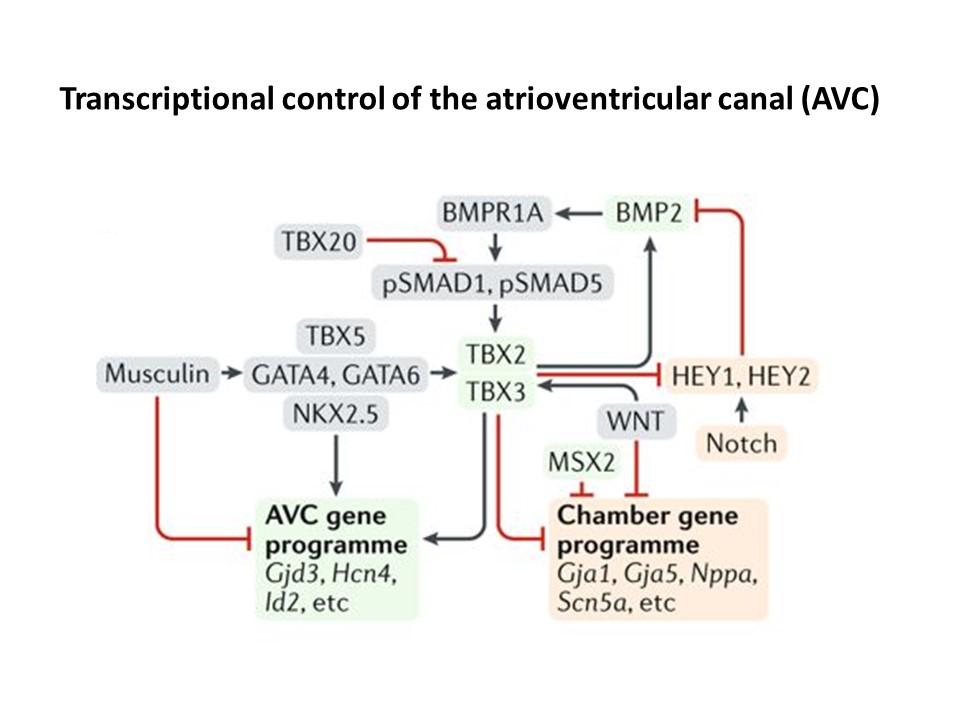
In addition to the development of pure pacemaker therapies, we are also determined to develop biological therapies that can repair dysfunctional atrioventricular (AV) conduction as this is among the most important causes underlying disease-causing bradycardia (slow heart rates). Our team therefore develops novel gene and stem cell therapies to repair AV-conduction. In this effort we collaborate closely with developmental biologists to implement their latest insights (see Figure 2). Together with the biological pacing program we anticipate these lines of research will generate a full spectrum of biological pacemaker solutions, applicable to virtually every pacemaker patient.
3. Prevention and treatment of common tachyarrhythmias
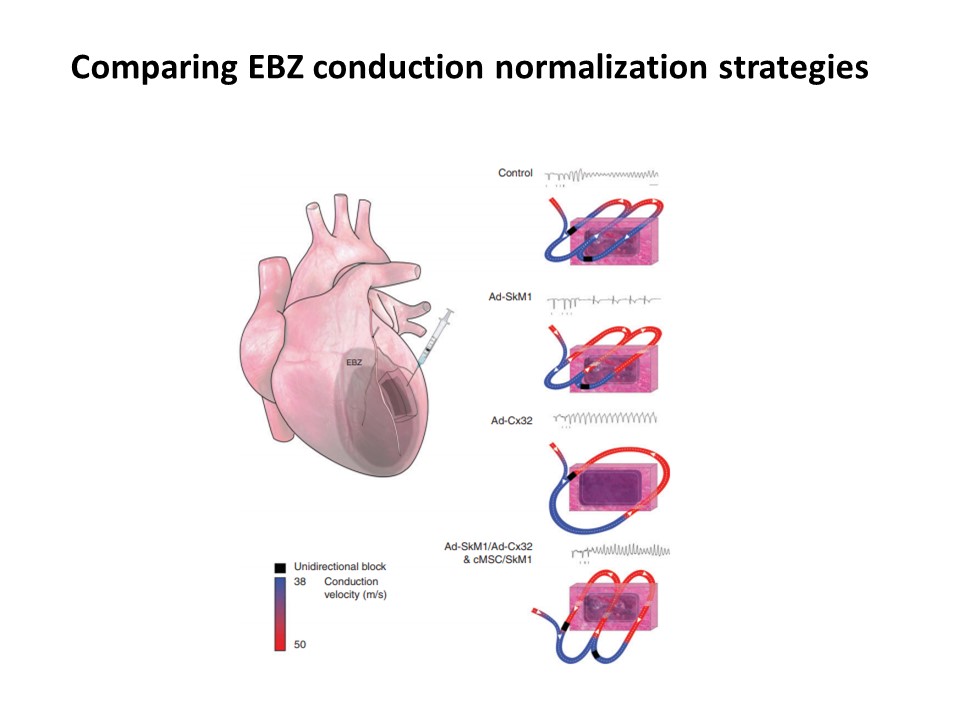
Sudden cardiac death constitutes a worldwide medical and social problem. Pharmacological prevention is often ineffective and may promote life-threatening arrhythmias. Implanted electronic cardioverter/defibrillators (ICDs) sometimes deliver inappropriate shocks resulting in serious health complications. They are also dependent on an implanted electronic energy source. We therefore develop gene and cell-based therapies restoring local electrical properties to prevent lethal arrhythmias. Figure 3 Summarizes various gene and cell therapy strategies for normalization of impulse propagation in the epicardial borderzone (EBZ) of infarcted ventricular myocardium. In this comparison viral delivery of the SkM1 ion channel was most effective in restoration of fast conduction and prevention of ventricular arrhythmias.
Figure 1. Mechanistic summary and comparison of basic parameters of the most promising biological pacemaker strategies. This comparison shows that ion channel-based biological pacing (in this case using HCN2/SkM1) can provide highly effective and hardware-independent function. See review for details: Boink et al. Trends Cardiovasc Med. 2015; 25:661-73.
Figure 2. Molecular mechanisms underlying atrioventricular canal (AVC) and heart chamber development. This cartoon highlights the different molecular pathways important for AVC formation. See review for details: van Eif et al. Nat Rev Cardiol. 2018.
Figure 3. Schematic cartoon of the infarcted heart treated with various viral and stem cell approaches to normalize conduction and modify the arrhythmogenic substrate. See review for details: Driessen et al. Europace. 2017; 19:518-528.
Gerard Boink (Group leader)
Dirk Geerts (Leader gene therapy program)
Rajiv Mohan (Post-doc)
Max Medina Ramirez (Post-doc)
Mischa Klerk (Technician)
Veronique Knaup (Technician)
Leontien Bosch (Technician)
Annemarie Vegh (PhD student)
Jiuru Li (PhD student)
Joseph Boink (Scientist)
Jianan Wang (PhD student)
Alexandra Wiesinger (PhD student)
Yu Li (PhD student)
Lotte Geerlings (Bachelor student)
Maikel van de Ven (Bachelor student)
Advisory Board
Dr. Hanno L. Tan (Cardiologist and Principal Investigator, AMC)
Prof. Dr. Vincent M. Christoffels (Developmental Biologist and Chairman Medical Biology, AMC)
Prof. Dr. Ir. Jacques M.T. de Bakker (Emeritus Professor of Experimental Cardiology, AMC and UMCU)
Prof. Dr. Michael R. Rosen (Gustavus A. Pfeiffer Professor of Pharmacology, Professor of Pediatrics, Columbia University, New York, U.S.A.)
Corporate and Business Development
Osne Kirzner (AMC)
Shobi Dhawan (AMC Ventures)
Jerry Kokoshka (Columbia Technology Ventures, New York, U.S.A.)
- van Eif VWW, Devalla HD, Boink GJJ, Christoffels VM. Transcriptional regulation of the cardiac conduction system. Nat Rev Cardiol. 2018. www.ncbi.nlm.nih.gov/pubmed/29875439
- Adepu S, Oosterwerrf FJ, Christoffels VM, Boink GJJ. Direct reprograming to regenerate myocardium and repair its pacemaker and conduction system . Medicines (Basel). 2018;5(2). www.ncbi.nlm.nih.gov/pubmed/29867004
- Smit NW, Cócera Ortega L, Végh AMD, Meijborg VMF, Smits AM, Klerk M, Tijsen AJM, Tan HL, Goumans MJHT, Boink GJJ, Coronel R. Human cardiomyocyte progenitor cells in co-culture with rat cardiomyocytes form a pro-arrhythmic substrate: evidence for two different arrhythmogenic mechanisms. Front Physiol. 2017;8:797. www.ncbi.nlm.nih.gov/pubmed/29075204
- Driessen HE, Boink GJJ, van Veen TAB. miRNA-based antiarrhythmics after myocardial ischaemic injury. Europace. 2017;19:518-528. www.ncbi.nlm.nih.gov/pubmed/28431070
- Driessen HE, van Veen TAB, Boink GJJ. Emerging molecular therapies targeting myocardial infarction-related arrhythmias. Europace. 2017;19:518-528. www.ncbi.nlm.nih.gov/pubmed/28431070
- Woudstra OI, Boink GJJ, Winkelman JA, van Stralen R. A Rare Case of Primary Meningococcal Myopericarditis in a 71-Year-Old Male. Case Rep Cardiol. 2016;2016:1297869. www.ncbi.nlm.nih.gov/pubmed/28003913
- Kiemeneij F, Boink GJJ. The PROPHET-II’s Prophecy. JACC Cardiovasc Interv. 2016;9:2000-2001. www.ncbi.nlm.nih.gov/pubmed/27712734
- Boink GJJ, Tan HL, Robinson RB, Cohen IS. The past, present, and future of pacemaker therapies. Trends Cardiovasc Med. 2015; S1050-1738. www.ncbi.nlm.nih.gov/pubmed/26001958
- Boink GJJ, Robinson RB, Gene therapy for restoring heart rhythm. J Cardiovasc Pharmacol Ther. 2014;19:426-38. www.ncbi.nlm.nih.gov/pubmed/24742766
- Costet A, Provost J, Gambhir A, Bobkov Y, Danilo P Jr, Boink GJJ, Rosen MR, Konofagou EE.. Electromechanical Wave Imaging of biologically and electrically paced canine hearts in vivo. Ultrasound in Medicine and Biology. 2014;40:177-87. www.ncbi.nlm.nih.gov/pubmed/24239363
- Xiao L, Koopmann TT, Ördög B, Postema PG, Verkerk AO, Iyer V, Sampson KJ, Boink GJJ, Mamarbachi MA, Varro A, Jordaens L, Res J, Kass RS, Wilde AA, Bezzina CR, Nattel S. Unique cardiac Purkinje fiber transient outward current β-subunit composition: a potential molecular link to idiopathic ventricular fibrillation. Circ Res. 2013;112:1310-22. www.ncbi.nlm.nih.gov/pubmed/23532596
- den Haan AD, Veldkamp MW, Bakker D, Boink GJJ, Janssen RB, de Bakker JM, Tan HL. Organ explant culture of neonatal rat ventricles: a new model to study gene and cell therapy. PLoS One. 2013;8:e59290. www.ncbi.nlm.nih.gov/pubmed/23516623
- Boink GJJ, Duan L, Nearing BD, Shlapakova IN, Sosunov EA, Anyukhovsky EP, Bobkov E, Kryukova Y, Ozgen N, Danilo P Jr, Cohen IS, Verrier RL, Robinson RB, Rosen MR. HCN2/SkM1 Gene Transfer Into Canine Left Bundle Branch Induces Stable, Autonomically Responsive Biological Pacing at Physiological Heart Rates. J Am Coll Cardiol. 2013;61:1192-201. www.ncbi.nlm.nih.gov/pubmed/23395072
- Özgen N, Lu Z, Boink GJJ, Lau DH, Shlapakova IN, Bobkov Y, Danilo P Jr, Cohen IS, Rosen MR. Microtubules and angiotensin II receptors contribute to modulation of repolarization induced by ventricular pacing. Heart Rhythm 2012;9:1865-72. www.ncbi.nlm.nih.gov/pubmed/22820054
- Boink GJJ, Nearing BD, Shlapakova IN, Duan L, Kryukova Y, Bobkov Y, Tan HL, Cohen IS, Danilo P Jr, Robinson RB, Verrier RL, Rosen MR. The Ca2+-stimulated adenylyl cyclase AC1 generates highly efficient biological pacing – as single gene therapy and in combination with HCN2. Circulation 2012 31;126:528-36. www.ncbi.nlm.nih.gov/pubmed/22753192
- Boink GJJ, Lu J, Driessen HE, Duan L, Sosunov EA, Anyukhovsky EP, Shlapakova IN, Lau DH, Rosen TS, Danilo P, Jia Z, Ozgen N, Bobkov Y, Guo Y, Brink PR, Kryukova Y, Robinson RB, Entcheva E, Cohen IS, Rosen MR. Effect of SkM1 sodium channels delivered via a cell platform on cardiac conduction and arrhythmia induction. Circ Arrhythm Electrophysiol. 2012;5:831-40. www.ncbi.nlm.nih.gov/pubmed/22722661
- Lu J, Wang HZ, Jia Z, Zuckerman J, Lu Z, Guo Y, Boink GJJ, Brink PR, Robinson RB, Entcheva E, Cohen IS. Improving cardiac conduction with a skeletal muscle sodium channel by gene and cell therapy. J Cardiovasc Pharmacol. 2012;60:88-99. www.ncbi.nlm.nih.gov/pubmed/22526298
- Bakker ML, Boink GJJ, Boukens BJ, Verkerk AO, van den Boogaard M, den Haan AD, Hoogaars WM, Buermans HP, de Bakker JM, Seppen J, Tan HL, Moorman AF, ‘t Hoen PA, Christoffels VM. T-box transcription factor TBX3 reprograms mature cardiac myocytes towards pacemaker-like cells. Cardiovasc Res. 2012;94:439-49. www.ncbi.nlm.nih.gov/pubmed/22419669
- Boink GJJ, Lau DH, Shlapakova IN, Sosunov EA, Anyukhovsky EP, Driessen HE, Dun W, Chen M, Danilo P Jr, Rosen TS, Őzgen N, Duffy HS, Kryukova Y, Boyden PA, Robinson RB, Brink PR, Cohen IS, Rosen MR. SkM1 and Cx32 improve conduction in canine infarcts, yet only SkM1 is antiarrhythmic. Cardiovasc Res. 2012;94:450-9. www.ncbi.nlm.nih.gov/pubmed/22374989
- Amin AS, Boink GJJ, Atrafi F, Spanjaart AM, Asghari-Roodsari A, Molenaar RJ, Ruijter JM, Wilde AA, Tan HL. Facilitatory and inhibitory effects of SCN5A mutations on atrial fibrillation in Brugada syndrome. Europace. 2011;13:968-75. www.ncbi.nlm.nih.gov/pubmed/21273195
- Boink GJJ, Rosen MR. Regenerative therapies in electrophysiology and pacing: introducing the next steps. J Interv Card Electrophysiol. 2011;31:3-16. www.ncbi.nlm.nih.gov/pubmed/21161675
- Shlapakova IN, Nearing BD, Lau DH, Boink GJJ, Danilo P Jr, Kryukova Y, Robinson RB, Cohen IS, Rosen MR, Verrier RL.Biological pacemakers in canines exhibit positive chronotropic response to emotional arousal. Heart Rhythm. 2010;7:1835-40. www.ncbi.nlm.nih.gov/pubmed/20708103
- Boink GJJ, Seppen J, de Bakker JM, Tan HL. Biological pacing by gene and cell therapy. Neth Heart J. 2007;15:318-22. www.ncbi.nlm.nih.gov/pubmed/18604282
- Boink GJJ, Verkerk AO, van Amersfoorth SC, Tasseron SJ, van der Rijt R, Bakker D, Linnenbank AC, van der Meulen J, de Bakker JM, Seppen J, Tan HL. Engineering physiologically controlled pacemaker cells with lentiviral HCN4 gene transfer. J Gene Med. 2008;10;487-97. www.ncbi.nlm.nih.gov/pubmed/18383475
Boink GJJ, Seppen J, de Bakker JMT, Tan HL. Gene therapy to create biological pacemakers. Med Biol Eng Comput. 2007;45;167-76. www.ncbi.nlm.nih.gov/pubmed/17048028 - Schalekamp T, Boink GJJ, Visser L, et al. CYP2C9 genotyping in acenocoumarol treatment: is it a cost-effective addition to international normalized ratio monitoring? Clin Pharmacol Ther. 2006;79;511-20. www.ncbi.nlm.nih.gov/pubmed/16765138