Gladka group – Cardioprotection and cardiac repair
Cardiovascular diseases (CVDs) are the number 1 cause of death globally. 17.9 million people die each year from CVDs, an estimated 31% of all deaths worldwide (source: World Health Organization). Despite the significant advances in therapies and prevention, mortality and morbidity are still high, and quality of life is poor. CVDs are chronic progressive conditions resulting in the inability of the heart to perform, leading to the development of pathological remodeling of the heart and subsequently heart failure (HF).
The research aims in our lab are to map the behavior, properties, and interactions of the individual cells of the heart during the progression of pathological remodeling to HF and to identify novel therapeutic targets for future interventions. We employ multiple approaches, including mouse genetics, single-cell approaches, AAV-mediated gene therapy and extensive validation of findings in cardiac tissues from patients with different etiologies of HF.
Contact: Monika M. Gladka m.m.gladka@amsterdaumc.nl

Ischemic heart disease
Heart failure (HF) due to ischemic heart disease (IHD) is a leading cause of morbidity and death worldwide. After an ischemic insult, patients develop a stiff fibrotic scar that hampers cardiac contractility, leading to HF. Since endogenous cardiac repair mechanisms of an adult mammalian heart are insufficient to repopulate lost myocardium and restore cardiac function, there is an urgent need to initiate cardioprotective mechanisms to minimize damage caused by ischemia and stimulate repair mechanisms in the already damaged heart.
ZEB2 is a transcription factor that plays a crucial role in heart repair
During our latest research, I identified the Zinc Finger E-box Binding Homeobox2 (ZEB2) transcription factor as an essential player during cardiac repair. We showed that ZEB2 is increased in stressed cardiomyocytes and induces cardioprotective crosstalk between cardiomyocytes and endothelial cells to enhance angiogenesis after MI. Cardiomyocyte-specific deletion of ZEB2 (Zeb2 cKO) resulted in impaired cardiac contractility and infarct healing after MI, while cardiomyocyte-specific ZEB2 overexpression (cardiomyocyte-specific ZEB2 transgenic – Zeb2 cTg) improved cardiomyocyte survival and cardiac function. We identified Thymosin b4 (TMSB4) and Prothymosin a (PTMA) as the main paracrine factors released from cardiomyocytes that stimulate angiogenesis by enhancing endothelial cell migration in vitro and in vivo (Gladka et al. 2021 Nat. Comm)
Recently we linked ZEB2 to oxidative stress showing that ZEB2 overexpression protects the heart from ischemic damage. Therefore, we aim to identify and validate novel therapeutic targets downstream of ZEB2 to protect the heart from ischemia-induced dysfunction, and coax these into new therapies that will improve the survival and quality of life of IHD patients. We closely collaborate with Prof. Christoph Maack from Wurzburg.
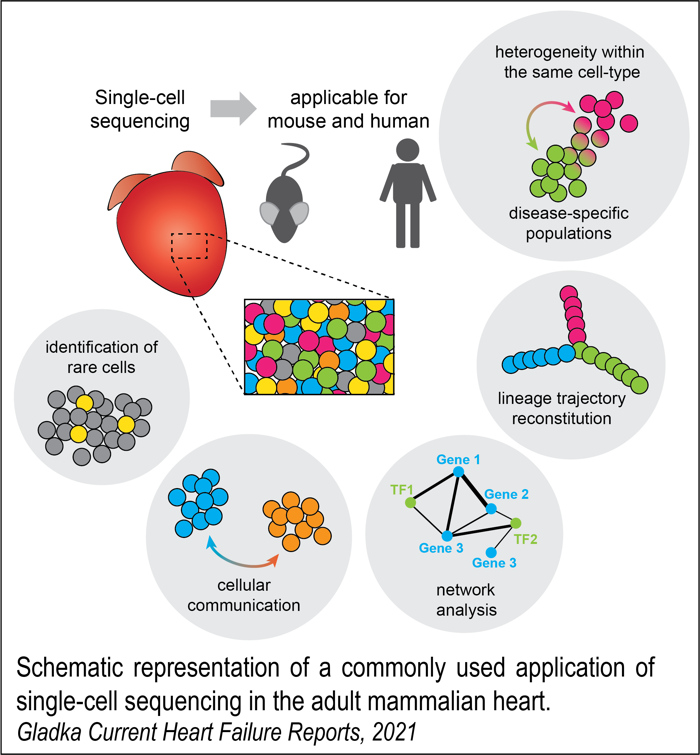
Single-cell RNA sequencing (scRNA-seq)
scRNA-seq has become a powerful tool for identifying cell types and studying gene expression changes in different tissues. Before developing this technology, researchers all over the world used traditional bulk sequencing methods to understand transcriptional differences in health and disease. The rapid deployment of scRNA-seq has revolutionized the field and resulted in the identification of previously unrecognized cell populations, including disease-specific cell states across a vast range of organs, including the brain, lung, liver, kidney, and heart.
We are applying scRNA-seq to healthy and diseased mouse and human hearts to identify heterogeneity among cell types and disease stages based on their transcriptional profile and examine imbalances in cell-type composition during cardiac stress and repair processes. We collaborate with Prof. Reinier Boon from Amsterdam UMN within the Out of the Box grant.
Mouse model of cardiac ischemia (IR)
IR injury is a major contributor to the cardiac damage caused by MI. To mimic this, we use a mouse model in which the left anterior descending artery (LAD) is surgically occluded and reopened to recapitulate the clinical situation. We use this model to study acute and chronic states of cardiac ischemia.
Gene therapy
We are using AAV-mediated gene transfer in vivo to test the therapeutic potential of newly identified factors. For this part, we closely collaborate with Prof. Mauro Giacca from Kings’ College in London and Dr Lorena Zentilin from Trieste.
Living cardiac slices
The lack of cardiac models that can replicate the human myocardium’s organ-level functioning hinders translational research in the field of cardiovascular medicine. Therefore, preclinical platforms that provide precise, standardized, lengthy, and rapid drug testing are in high demand. Heart slice culture is an emerging technology that solves many problems related to conventional myocardial culture systems. Heart slices have recently been used in several studies, demonstrating their ability to maintain the adult phenotype in a multi-cell environment for long periods. This technology is currently being optimized in our lab. We are able to culture cardiac slices from adult pig hearts for several days. The figure below shows an overview of the setting we currently use in pigs. Our long-term goal is to use this technology in the future in human hearts from donors we have access to.
 |
| Monika Gladka – Group Leader
Monika joined the department of Medical Biology as an Assistant Professor in 2021. Her current research focuses on understanding the molecular mechanisms that regulate cardiac repair, intending to identify new players to develop novel, improved gene therapies. She uses several state-of-the-art techniques such as single-cell sequencing, enabling an in-depth mechanistic understanding of the biological processes in injured hearts. Dr M. Gladka has 13 years of experience in molecular biology and animal studies. She did her PhD in the lab of Prof. L.J. de Windt, where she studied transcriptional and post-transcriptional gene regulation during heart failure. After that, she joined the lab of Prof. E. van Rooij where she focused on understanding the molecular mechanisms of cardiac regeneration with the ultimate goal of developing new therapies. In 2016 and 2020, she received two prestigious Dr E. Dekker personal grants from the Dutch Heart Foundation for heart repair research. In addition, she is actively involved in several cardiac societies acting as a board member of Young@Heart from the Netherlands Heart Institute and a nucleus member of the Scientists of Tomorrow from the European Society of Cardiology. |
Collaborating researchers
 |
 |
| Dr. Maurice van den Hoff | Prof. dr. Roelof-Jan Oostra |

Ing. Jaco Hagoort (j.hagoort@amsterdamumc.nl)
I am a research technician and responsible for the image acquisition, modeling and visualization methods that we use in the 3D projects. I enjoy to find and apply new tools and techniques, to make medical and biological findings easier to understand. Traditionally we do this with images of histological serial sections, Amira software and 3D-PDFs. But by combining this with contrast enhanced micro-CT, smarter segmentation, online visualization and collaboration with students and experts, I think we are able to create a valuable and easy accessible source of knowledge.

Ing. Quinn Gunst (q.d.gunst@amsterdamumc.nl)
I am a research technician and I take care of the laboratory, making sure that I can help as many people as I can with their questions in research and discovering new techniques to be able to do so. I try to do this by acquiring different techniques and explaining this to others, this can range from a bachelor student to a post-doc in the field. This part is what I love about my job, explaining, helping and assisting people to reach their goals. Histology and Qpcr are the main fields where I am active in and I hope I will be able to do so for as long as possible.
PhD students

Yousif Dawood, MD (y.dawood@amsterdamumc.nl)
3D atlas of human fetal anatomy
During my PhD I am mainly focusing on the human fetal development. To do so, we use fetuses from the Dutch Fetal Biobank to create an interactive 3D atlas and database of human fetal anatomy that comprises the complete human morphological development from 10 to 24 weeks of pregnancy. With the use of the newest imaging modalities, e.g. ultra-high field MRI and micro-CT, and our state of the art staining protocols, we are able to visualize fetal development on microscopic scale. Out of these visualizations and with the help of tens of students, we are creating models of each and every organ system. Our future perspective is to use these models for educational, research and clinical purposes.

Marieke Buijtendijk, MD (m.f.buijtendijk@amsterdamumc.nl)
A 3D ultrasound atlas of fetal development
Congenital anomalies affect about 30 per 1000 live-born children and are a leading cause of neonatal mortality. Early detection of these conditions is vital, and can be achieved through ultrasound screening. However, only about half of all anomalies are currently detected. Recent advances in three-dimensional (3D) ultrasound provide a novel way of assessing fetal anatomy. Its use in clinical practice is limited through the lack of an appropriate reference.
In this study, we examine how fetal anatomy is visualized on 3D ultrasound imaging using novel volume rendering technologies. Healthy fetuses donated to science following termination of pregnancy are imaged consecutively by 3D ultrasonography and micro-computed tomography (micro-CT). Using micro-CT imaging as a reference standard, we can validate the structures appearing on the rendered 3D ultrasound volumes. These datasets are then matched to previously obtained 3D ultrasound scans of healthy fetuses imaged in utero to test the applicability of our findings to the clinical situation. Through this project we will create a unique reference work on 3D ultrasonography and enhance our understanding of the potential of novel 3D ultrasound technologies for the assessment of fetal anatomy.

Hans Smit, MD (j.a.smit@amsterdamumc.nl)
Embryology, diagnosis and treatment of cleft lip and palate
Palatogenesis is a complex spatiotemporal process including development and fusion of the primary and secondary palatal shelves. The complexity of this process reflects the high incidence of cleft palates in humans (~1 : 2,500 live births). In 50% of cleft palate, the cause is non-syndromic. In these cases, etiology is complicated and not yet completely understood.
Increase of embryological knowledge is crucial in adequate cleft surgery. To facilitate understanding, we are adding a 3D reconstruction of palatogenesis in different Carnegie stages in addition to the already existing ‘3D Atlas of Human Embryology’. Our cleft chapter serves both educational and clinical purposes.

Matthijs Fockens, MD (m.m.fockens@amsterdamumc.nl)
Developmental aspects of laryngeal and tracheal anomalies
My research focuses on developmental and clinical aspects of laryngeal and tracheal anomalies. These anomalies generally present in neonates with respiratory complaints like stridor, dyspnea and cyanosis, and are accountable for morbidity and mortality. The exact cause of most laryngeal and tracheal anomalies are unknown, although the development of airway cartilage seems to play an important role.
Research topics include a descriptive analysis of normal tracheal cartilage development in human embryos, an evaluation of treatment modalities for airway obstruction caused by a third or fourth branchial pouch sinus, an assessment of a new surgical technique for aortopexy in tracheobronchomalacia, and a review of tracheal anomalies in Down syndrome children.

Malou Lugthart, MD (m.a.lugthart@amsterdamumc.nl)
Impact of prenatal screening: facing clinical challenges, improving counseling for our patients
Prenatal detection of fetal anomalies is considered to be an important goal of obstetric care and in many countries fetal ultrasound is now an established part of routine care. Routine screening and subsequent prenatal detection of fetal anomalies provides parents with the option to make autonomous reproductive choices. In this thesis, part I will address chromosomal anomalies, focusing on the prenatal detection and management of triploidy and describing the survival of trisomy 13 and 18. Part II will address structural anomalies in first and mid-trimester pregnancy: is there a reason for referral in case of an increased nuchal translucency before 11 weeks of gestation? And what is the impact of the first trimester scan on prenatal detection of structural anomalies and in particular congenital heart disease.

Valérie Niehe, MD (valerie.niehe@ghz.nl)
Forensic perinatal and pediatric radiology
Topics:
- Fetal age estimation: which method should be used in the forensic radiologic setting?
Systematic literature review, set up a guideline for the estimation of fetal age in a forensic setting, is it feasible to make an (open source) tool for the estimation of fetal age?
- Use of micro-CT in the perinatal and pediatric forensic setting.
Systematic literature review. Use of micro-CT in the setting of perinatal and pediatric radiology?
- Case study of neonaticides. Is it possible to determine if a fetus has lived after birth using total body CT? How reliable are radiologic signs for the determination if a fetus has lived after birth (e.g. air in lungs, trachea, bronchial tree, gastrointestinal tract)? Comparison to autopsy findings.
- Prospective study of value of MRI in addition to CT and skeletal survey of children up to the age of 2 years in a forensic setting. Neuro / body radiology.
Özlem Engin, MD (o.engin@amsterdamumc.nl)
Surgical anatomy of the orbital apex
My research focusses on the anatomy of the orbital apex. We recently published a systematic review about the currently known anatomy. Now we will study the surgical anatomy of the orbital apex with a micro CT-scan. Hereafter we will study the Annulus of Zinn and it’s relationship with the optic nerve, the its precise location. After this we will look into the surgical anatomy from the supero- lateral surgical corridor to the orbital apex and skull base, using both an external approach and trans- orbital endoscopic approach. We will re-evaluate the orbital fibrous septae described by Prof. dr. Leo Koornneef and finally describe the orbital septae in the apex.
Wall of Fame of Former PhD students

Dr. Henri M. de Bakker
Forensic Radiology in the Netherlands
Click here for an updated list of all PubMed indexed references.
Additional publications
- Soerdjbalie V, de Bakker BS, de Bakker HM. Symbiotische samenwerking tussen patholoog en radioloog; 2020 Sept;25(3):7-8.
- de Bakker HM, Tijsterman M, de Bakker-Teunissen OJGB, Soerdjbalie-Maikoe V, van Hulst RA, de Bakker BS. Prevalentie van bullae en blebs bij post-mortem CT. Implicaties voor duikgeneeskunde?; 2020 Sept;25(3):12-17.
- Sonnemans L, Dawood Y, Klein W, de Bakker BS. Postmortale beeldvorming van foetussen; 2020 Sept;25(3):30-35.
- de Bakker HM, Oltshoorn PC, Soerdjbalie-Makoe V, de Bakker BS. The most accurate postmortem radiological imaging method to evaluate suspected neck violence; Forensic Imaging Accepted March 31, 2020
- de Bakker HM, de Bakker BS. Forensic radiology in the Netherlands: a personal perspective. Journal of Forensic Radiology and Imaging. Volume 9, June 2017, 56–58.
- de Bakker HM, Soerdjbalie-Maikoe V, Kubat B, Maes A, de Bakker BS. Forensic imaging in legal medicine in the Netherlands: Retrospective analysis of over 1700 cases in 15 years’ experience. Journal of Forensic Radiology and Imaging. Sept 2016, Volume 6, 1–7.
- de Bakker BS, Soerdjbalie-Maikoe V, de Bakker HM. The use of 3D-CT in weapon caused impression fractures of the skull, from a forensic radiological point of view. Journal of Forensic Radiology and Imaging, Oct 2013;1(4):176–179.
Book chapters
- DeRuiter, MC, Kleinrensink, G & de Bakker, BS (2019). Anatomy of the female pelvis, pelvic organs & reproductive system. In: EAP Steegers e.a. (red). Textbook of obstetrics and gynaecology. A life course approach. Houten: Bohn Stafleu van Loghum.
- Ganzevoort, W, Van Wassenaer-Leemhuis, AG, Painter, RC, Steegers-Theunissen, RPM, & De Bakker, BS (2019). Embryonic & fetal growth and development. In: EAP Steegers e.a. (red). Textbook of obstetrics and gynaecology. A life course approach. Houten: Bohn Stafleu van Loghum.
Cover images
Since 2017, parents can donate a fetus to science after termination of pregnancy, an ectopic pregnancy or immature labor below 24 weeks of gestation (the limit of viability). This biobank at Amsterdam UMC, (location AMC) is treated with the greatest care, respect and anonymity. When parents have decided to terminate their pregnancy, their wishes for the destination of the body of the fetus after birth are discussed by their obstetrician or midwife. In hospitals that are affiliated to the fetal biobank, parents are given the option to donate the fetus to science, in addition to the standard options of taking the fetus home for a private burial or cremation, or collective cremation organized by the hospital. When parents decide to donate their fetus to science after birth, procedures during the labor and birth are as usual. After the birth, parents can take all the time they need to say goodbye. When they have given their permission for the fetus to be collected, a member of the biobank will be alerted and the fetus will be transported to the biobank as soon as possible (24/7). Once parents have donated the fetus, the fetus can no longer be traced back to the parents, as such anonymity is guaranteed.
After researchers from the biobank have collected the fetus, it will be scanned using 3D ultrasound, 7 Tesla MRI or Micro-CT to study the anatomy of organs and to validate new ultrasound screening software. Subsequently, samples that are useful for DNA, RNA and protein studies are taken from all organs. Brain tissue is stored in the national Netherlands Brain Bank. Using the fetal tissues, researchers within our group investigate gene expression pathways and how the various organs develop. This increases our understanding of normal growth as well as how birth defects might occur.
When parents wish to donate a fetus that died after 24 weeks of gestation to science, can do so through the body donation program.
For questions concerning the fetal biobank please email at foetale.biobank@amsterdamumc.nl.
Lead scientists
Dr. Bernadette de Bakker
Dr. Maurice van den Hoff
Affiliated hospitals
Amsterdam UMC, location AMC
OLVG (East en West)
Spaarne Gasthuis (Hoofddorp & Haarlem)
Rode Kruis Ziekenhuis
Zaans Medisch Centrum
Noord West Ziekenhuisgroep (Alkmaar & Den Helder)
Flevoziekenhuis
Tergooi Ziekenhuis
Erasmus MC
Amsterdam Reproduction & Development (AR&D)
PhD Thesis: 3D Atlas of Human Embryology – New insights in human development
Outreach and publicity
The societal impact of our work is evident from the fact that multiple international media platforms have featured our work including the BBC, CNN, Fox news, The New York Times, The Guardian, NRC and Het Parool. Just recently, our discovery of a new salivary gland (Valstar, de Bakker et al.Oct.2020) went viral. We have published several articles in scientific layman’s journals like ‘Hoe bouw je een baby’ KIJK(2019);10:24-29, contributed to the latest exhibition HUMANIA of NEMO Science Museum in Amsterdam (21-11-2019) and have acted in a documentary by Medialogica(NPO2) – De beeldenstorm rond abortus – (08-12-2019).
I work as lecturer in the Section Clinical Anatomy & Embryology of the dept. of Medical Biology. We teach human anatomy and embryology to bachelor, master and post-doctoral students for multiple faculties of the University of Amsterdam. My current research portfolio encompasses the following courses.
Coordinator:
- Forensic Medicine – Faculty of Medicine, University of Amsterdam (18 EC)
- Anatomy & Developmental Biology – Faculty of Science, University of Amsterdam (12 EC)
Other courses I am currently enrolled in:
- Shaping in a Human, track Developmental & Therapeutic Biology, master Biomedical Scienses, Faculty of Science, University of Amsterdam
- Human Body II, Bachelor Amsterdam University College, Faculty of Science, University of Amsterdam
- 3D Atlas of Human Fetal Anatomy elective course, Faculty of Medicine, University of Amsterdam
- RA-1 (Regulation and Immunity 1), Faculty of Medicine, University of Amsterdam
- RA-3 (Regulation and Immunity 3), Faculty of Medicine, University of Amsterdam
- General and Applied Embryology – Faculty of Medicine UMC Utrecht, University of Utrecht
- Minor Challenges in Womens and Child Healthcare, Faculty of Medicine, VU University
- Surgical Anatomy for surgeons in training – Amsterdam UMC
- Gynecological Anatomy for gynecologists in training – Amsterdam UMC
- Basic course prenatal ultrasound, EchoXpert academy Amsterdam
Supervising:
I have supervised 133 students (as per January 2021) through various individual internships. Check the TEAM page to find out about the PhD students I currently supervise.
Funding
Funding for our research was kindly provided by the following organizations:
- The Board of Directors of the Amsterdam UMC
- Amsterdam Reproduction & Development (AR&D)
- De Snoo-van ’t Hoogerhuijs Foundation
- Innovation Exchange Amsterdam (IXA)
- Samsung Healthcare
Awards, Prizes and Grants
- Amsterdam UMC Innovation impuls: Augmented Reality for education about cleft lips and palates (2021)
- AR&D The Bigger Picture Grant (2020)
- AR&D In Between Grant (2020)
- The AMC Best PhD Thesis Award (2019)
- Amsterdam Science & Innovation award (2019)
- Young investigator award by De Snoo-van ‘t Hoogerhuijs foundation (2019)
- IXA and Samsung: An atlas of 3D ultrasound images of human development (2018)
- AR&D Out-of-the-Box grant (2018)
- Out-of-the-box grant Cardiovascular Sciences (2018)
- Best Amsterdam Cardiovascular Sciences Publication Award (2017)
- AMC Graduate School Best PhD Publication Award (2017)
- AMC Graduate School PhD Scholarship for Yousif Dawood (2017)
- AMC Grassroot (2017)
- AMC Profile prize 3D Atlas Team (2017)
- AMC Innovation impuls: ACRA: an anatomic-radiological skills lab (2016)
- IPS-project Amsterdam University Fund; Serious gaming (2015)
- American Association for Anatomy travel award (2014)
- Bolk Award of the Dutch Anatomical Association (2013)
- American Association for Anatomy Visiting Scholarship (2013)
- American Association for Anatomy travel award (2013)
- IXA Proof of concept funding for the development of the 3D Atlas Application (2012)
- American Association for Anatomy travel award (2012)
- Spinoza funding of the University of Amsterdam (2011)
- AMC Best Honours Student Presentation Award (2009)